分享到
Prediction of the Key Binding Site of Odorant-binding Protein of Holotrichia oblita
The scarab beetle Holotrichia oblita Faldermann (Coleoptera: Scarabaeidae) is a predominant underground pest in the northern parts of China, and its larvae (grubs) cause great economic losses because of its wide range of host plants and covert habitats. One potential pest management measure is the regulation of olfactory chemoreception in beetles to control larvae. In the process of olfactory recognition, odorant-binding proteins (OBPs) are believed to carry hydrophobic odorants from the environment to the surface of olfactory receptor neurons. Recently, researchers at the State Key Laboratory for Biology of Plant Diseases and Insect Pests, Institute of Plant Protection, CAAS have published a research paper about the key binding site of HoblOBP with its ligands.
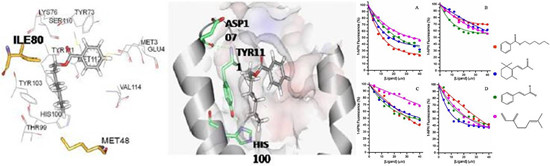
(1)The binding pocket of HoblOBP1 and the docking result with hexyl benzoate.(2)Competitive binding curves of selected ligands to the mutants and original protein of HoblOBP1.
In this paper, the authors first briefly proposed that controlling the adult is a simple and effective way because of grub’s variety and covert behavior. And then, the paper emphasized that understanding the molecular mechanism for plant host recognition mediated by olfaction would help in developing new strategies for the control of H. oblita. Based on the previous research, homology modeling of HoblOBP1 has been conducted and the three-dimensional structure of HoblOBP1 contains six α-helices and three disulphide bridges that connect the helices, and the hydrophobic pockets are both composed of five helices. The results of molecular docking with hexyl benzoate showed that van der Waals interactions and hydrophobic interactions are both important in the bonding. Intramolecular residues formed the hydrogen bonds in the C terminus of the protein and the bonds are crucial for the ligand-binding specificity. MET48, ILE80 and TYR111 are the predicted binding sites for HoblOBP1. Site-directed mutagenesis and fluorescence assay demonstrated that ligands could not be recognized by mutant of Tyr111. A possible explanation is that the compound could not be recognized by the mutant, and remains in the binding cavity because of the loss of the intramolecular hydrogen bonding that acts as a holder. So the authors believe that Tyr111 of HoblOBP1 is a key binding site. They also believe that Ile80A is a very important binding site, especially to some ligands.
More details are available on the bellow links:
http://onlinelibrary.wiley.com/doi/10.1111/imb.12088/full

(1)The binding pocket of HoblOBP1 and the docking result with hexyl benzoate.(2)Competitive binding curves of selected ligands to the mutants and original protein of HoblOBP1.
In this paper, the authors first briefly proposed that controlling the adult is a simple and effective way because of grub’s variety and covert behavior. And then, the paper emphasized that understanding the molecular mechanism for plant host recognition mediated by olfaction would help in developing new strategies for the control of H. oblita. Based on the previous research, homology modeling of HoblOBP1 has been conducted and the three-dimensional structure of HoblOBP1 contains six α-helices and three disulphide bridges that connect the helices, and the hydrophobic pockets are both composed of five helices. The results of molecular docking with hexyl benzoate showed that van der Waals interactions and hydrophobic interactions are both important in the bonding. Intramolecular residues formed the hydrogen bonds in the C terminus of the protein and the bonds are crucial for the ligand-binding specificity. MET48, ILE80 and TYR111 are the predicted binding sites for HoblOBP1. Site-directed mutagenesis and fluorescence assay demonstrated that ligands could not be recognized by mutant of Tyr111. A possible explanation is that the compound could not be recognized by the mutant, and remains in the binding cavity because of the loss of the intramolecular hydrogen bonding that acts as a holder. So the authors believe that Tyr111 of HoblOBP1 is a key binding site. They also believe that Ile80A is a very important binding site, especially to some ligands.
More details are available on the bellow links:
http://onlinelibrary.wiley.com/doi/10.1111/imb.12088/full
Latest News
-
 Apr 18, 2024Opening Ceremony of the Training Workshop on Wheat Head Scab Resistance Breeding and Pest Control in Africa Held in CAAS
Apr 18, 2024Opening Ceremony of the Training Workshop on Wheat Head Scab Resistance Breeding and Pest Control in Africa Held in CAAS -
 Apr 03, 2024IPPCAAS Co-organized the Training Workshop on Management and Application of Biopesticides in Nepal
Apr 03, 2024IPPCAAS Co-organized the Training Workshop on Management and Application of Biopesticides in Nepal -
 Mar 28, 2024Delegation from the School of Agriculture and Food Science of University College Dublin, Ireland Visit to IAS, CAAS
Mar 28, 2024Delegation from the School of Agriculture and Food Science of University College Dublin, Ireland Visit to IAS, CAAS -
 Mar 25, 2024Director of World Food Prize Foundation visited GSCAAS
Mar 25, 2024Director of World Food Prize Foundation visited GSCAAS -
 Mar 20, 2024Institute of Crop Sciences (ICS) and Syngenta Group Global Seeds Advance Collaborative Research in the Seed Industry
Mar 20, 2024Institute of Crop Sciences (ICS) and Syngenta Group Global Seeds Advance Collaborative Research in the Seed Industry
