Advances in nucleic acid-based reference materials: a "ruler" for precision molecular diagnostics
Recently, the Innovation Team of Agricultural Reference Materials from the Institute of Quality Standards and Testing Technology for Agro-Products of CAAS has published a paper titled "Nucleic acid-based reference materials: a 'ruler' for precision molecular diagnostics" in the international authoritative journal TrAC Trends in Analytical Chemistry. The paper comprehensively and deeply elaborates on the latest progress in nucleic acid reference materials (NARMs).
NARMs, as the "gold standard" to ensure the accuracy and traceability of nucleic acid test results, have become increasingly important in the field of molecular precision diagnosis. The Innovation Team of Agricultural Reference Materials systematically introduces the classification of NARMs, elaborates the characterization method, and highlights their application in key areas such as virus diagnosis, animal disease detection and GMO detection.
The paper also focuses on the analysis of NARMs in future application scenarios such as high-precision sequencing, tumor markers and non-coding RNA, with a view to providing prospective strategies for cross-field synergistic innovation and clinical application, and assisting the standardization and precision development of molecular diagnostic technologies.
At the same time, the author also pointed out that the current types and quantities of NARMs cannot fully meet the needs of society, especially in the fields of tumor biomarkers, circRNAs and miRNAs, the contradiction between supply and demand is particularly prominent. To address this issue, future efforts should focus on the field of in vitro diagnostic high and new technology, oriented by the actual clinical needs, integrating genomics, transcriptomics and proteomics and other multi-omics technologies, developing composite NARMs with multi-dimensional features, and realizing the precise quality control of nucleic acid molecules with multiple feature values. Close collaboration among metrology organizations, regulatory authorities, testing laboratories, instrument manufacturers and other relevant departments will be strengthened to provide core support for precision medicine, prevention and control of emerging infectious diseases and biosafety governance.
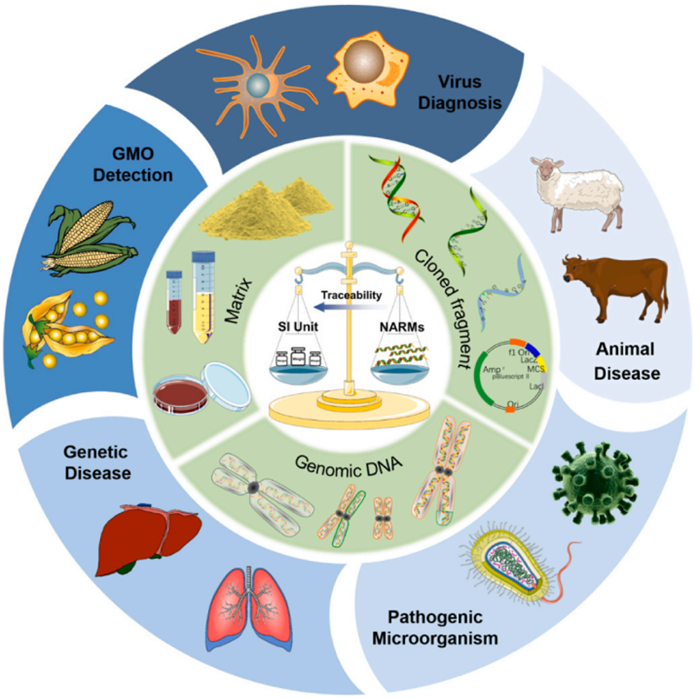
Figure 1: A summary of classifications and applications of NARMs.
Link: https://doi.org/10.1016/j.trac.2025.118268.
By Li Kai
(likaij@163.com)
-
 Apr 25, 2025CAAS President Meets Director General of IAEA
Apr 25, 2025CAAS President Meets Director General of IAEA -
 Apr 25, 2025CAAS Delegation Attends CGIAR Science Week 2025
Apr 25, 2025CAAS Delegation Attends CGIAR Science Week 2025 -
 Apr 25, 2025Delegation Led by CEO of Punjab Agricultural Research Council Abid Mahmood and Vice Dean of PKU Advanced Agricultural Research Institute Zhang Xingping Visits Shouguang R&D Center of CAAS
Apr 25, 2025Delegation Led by CEO of Punjab Agricultural Research Council Abid Mahmood and Vice Dean of PKU Advanced Agricultural Research Institute Zhang Xingping Visits Shouguang R&D Center of CAAS -
 Apr 11, 2025Strengthening China-Mongolia Grassland Cooperation to Build a Cross-Border Green Ecological Barrier
Apr 11, 2025Strengthening China-Mongolia Grassland Cooperation to Build a Cross-Border Green Ecological Barrier -
 Apr 03, 2025CAAS and CABI Forge a New Chapter in Strategic Cooperation
Apr 03, 2025CAAS and CABI Forge a New Chapter in Strategic Cooperation
